Healthmirror
Healthmirror
is a platform design that provides fast access to real world data
in Phases III and IV of clinical development in Europe,
starting with vaccines against COVID-19
Under development by nxt statista, the project unit of the global business data platform Statista
Healthmirror
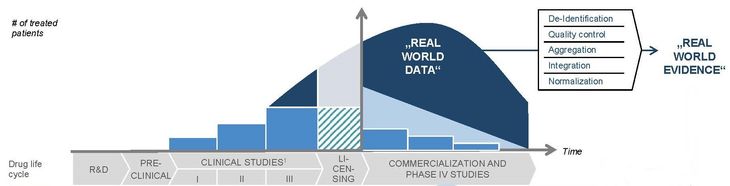
Proving the efficacy of vaccines in randomized controlled trials, especially at the advanced clinical development phases is fraught with difficulty. There are three main reasons for this. These include technical and ethical issues surrounding controlled human infection models, test environments with very low infection rates of the virus to be vaccinated against, and the need for rigorous long-term testing. Vaccines will usually receive a conditional market authorization with the requirement for post-authorization studies based on real world data. However, mainly due to regulatory issues, for a very wide range of test cases, this data is difficult to obtain in Europe, or is acquired only after a considerable delay.
The Healthmirror platform supports observational post-authorization effectiveness and safety studies for vaccines and therapeutics by facilitating real time access to real world data from Electronic Health Records (EHRs). For this purpose, anonymized treatment data from the primary care information systems in several European countries are transferred to Healthmirror on a daily basis. Healthmirror compares the state of health of vaccinated and non-vaccinated groups and detects when the virus reappears in the vaccinated group or when adverse reactions start to appear in particular subgroups of the vaccinated population. Multi-stage anonymization algorithms and transparent registration processes for the participating physicians ensure that the platform complies with data protection regulations like GDPR.
The Healthmirror platform approach is developed together with technology partners like CompuGroup Medical, the leading provider of Practice Management Systems in Europe.
Using real world evidence to make vaccine development safer and more effective
our insight of the week - June 26, 2020
Dr. Thilo Löwe. photo courtesy LSP
Right now, multiple candidates for a vaccine against Covid-19 are being developed all around the world. The point in time when a vaccine becomes available marks, for many people, the time we might be able to “go back to normal”. But contrary to common belief, the work will not stop there. For Healthmirror and Thilo Löwe, this is when the real work starts.
Healthmirror is a project currently under development by nxt statista, the innovation hub of the German company Statista, one of the most used statistical databases in the world. Therefore, Thilo Löwe, nxt statista’s Managing Director, knows a lot about the power of data – and the lack thereof. In the case of COVID-19 vaccines, regulators are likely to authorize a new vaccine in a fast track process as soon as the safety of the vaccine and initial proof of efficacy have been established in clinical trials, but they will also demand so-called post marketing studies to assess the effectiveness of the vaccine in the real world, outside a trial environment, when it is administered to a broad population.
The problem with these studies, Löwe explains, is that by the time a vaccine will be authorized and finally administered to the population, the Covid-19 infection rates will probably – hopefully, he adds – be very low, due to the widespread hygiene and social distancing measures being implemented. While a low infection rate is obviously a desirable state, it makes testing the vaccines’ efficacy a lot more difficult: how can you tell if it is really working for everyone if a large proportion of the people who were vaccinated never come into contact with the virus?
“You can only solve this dilemma with a monitoring system, tracking the effectiveness of the vaccine after it has been administered, not retrospectively, but as a continuous counterpart of the vaccination process”, says Löwe. That is what Healthmirror is aiming at: creating a platform collecting vaccination data in real time. The data is received from a panel of general practitioners who, in most European countries, are the ones administering vaccines and would also be the first ones to find out if their patients showed symptoms of Covid-19 even after being vaccinated.
Ideally, the panel would contain around 3.000 doctors per country who send out anonymized data from their electronic patient files. A general practitioner sees around 800 patients per quarter, so the database could keep track of around 2.4 million people per country. To get this kind of range, Healthmirror will be partnering with leading practice management systems in Europe. General practices already use this type of software for keeping patient files, therefore, participating in the panel would not require any extra effort or a new processes on their side.
All the participating doctors’ patients receive an anonymous ID, so it is impossible to trace a single patient, and only de-identified data is being transmitted. These steps are important for data protection and still allow Healthmirror to gain substantial insight. Even if they can’t contact a Covid-19 patient who fell ill after vaccination to find out more about their situation, they can still use the anonymized data to compare the case to others and look for emerging patterns in age, sex, preexisting conditions or interactions with other medication, for example.
Insight on every possible correlation is something that clinical studies prior to authorization just cannot gain, Löwe explains. Prior to licensure of a new drug, it is impossible to include every imaginable type of patient in a trial, especially when it comes to overlapping effects. A sex or age balance in a trial’s test group can be achieved, but including someone who for example is pregnant, has specific allergies and also takes medication against high blood pressure is not as easy. If it turns out that any combination of these factors has an impact on the effectiveness or safety of the new drug, the clinical trial could potentially miss it. But over time, Healthmirror could capture this data and detect the correlation.
“We want to be ready as soon as the first vaccines are being authorized”, says Löwe. By then, Healthmirror will know exactly what kind of documentation the regulators will require from vaccine manufacturers and will be able to adapt their tools accordingly. And it doesn’t end there. “The idea is to have a platform for multiple use cases, not just for different Covid-19 vaccines but other diseases as well”, says Löwe. Eventually, he would like to see Healthmirror working with other international platforms to create a global system that uses data to assure the safety and efficacy of vaccines and other therapeutics.
June 26, 2020 by kENUP