Amidst worries about a third COVID-19 wave and emerging coronavirus variants, there’s a lot of good news from Italy: 2 clinical trials have started on the same day.
On March 1, Takis and Rottapharm Biotech announced the start of the clinical trial of COVID-eVax, their jointly developed vaccine candidate. "The start of the clinical trials represents an important step in the development of DNA technology against COVID-19 but also for other diseases", says Luigi Aurisicchio, CEO and scientific director of Takis.
But that is not all, AchilleS Vaccines, that was granted financing in June by the EU Malaria Fund, together with Toscana Life Sciences (trial sponsor) and Cross Alliance (a CRO) is managing clinical trial sites on Monday for their monoclonal antibody, mAbCo19. “Within a bit more than six months we’ve been able, with the support of an outstanding team, to transform a patent into a product which is quite an unbelievable outcome”, says Riccardo Baccheschi, CEO and president of AchilleS Vaccines. “The aim is now reached; the product is there.”
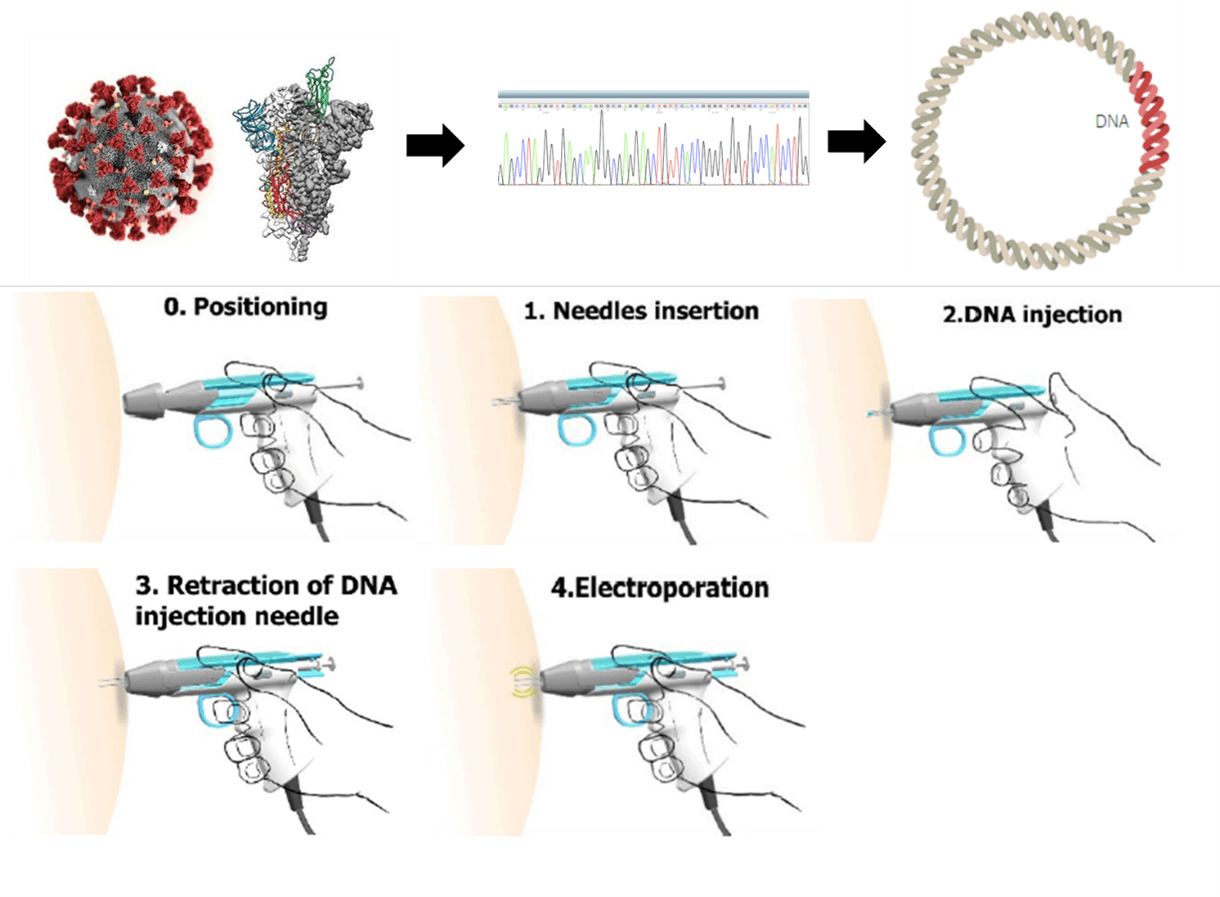
Unlike other vaccines already on the market, COVID-eVax is based on DNA. When injected into the muscle the DNA fragment promotes the production of a specific portion of the “spike” protein – the one the coronavirus uses to attach to human cells. Those portions of the “spike” protein produced by human cells then stimulate a strong immune reaction against the virus. In the case of COVID-eVax, the efficiency of the process is increased by “electroporation”, light and short electrical stimuli that facilitate the passage of DNA into the cells. That’s why Takis calls it a “precision medicine vaccine” approach.
A Phase 1 and 2 trial that provides results on safety and immunogenicity has now started in three hospitals: San Gerardo in Monza, Istituto Lazzaro Spallanzani in Rome and Istituto Pascale in Naples. As Luigi Aurisicchio explains in a video call there will be four different cohorts of patients. Three of them will be vaccinated on a prime-boost modality meaning they will get two injections of 0,5 milligram, 1,0 milligram or 2 milligrams of DNA respectively. Since the WHO recommends exploring a vaccine that works with a single administration, the fourth cohort in the COVID-eVax trials will receive only one injection of 2 milligrams of DNA.
Just a stone’s throw away in Siena, scientist Rino Rappuoli and his team found the perfect monoclonal antibody candidate. Normally, monoclonal antibodies are administered intravenously. The process can take hours. “Due to the particular need of COVID-19 pandemic, we wanted to produce an intramuscular product and avoid patient hospitalization for treatment purposes. A hospitalization which is necessary with products like those of Regeneron or Lilly’s” says Riccardo Baccheschi. MAbCo19 comes in a vial of 2,5ml that can be given to a patient in a single administration. What the optimal dosage might be the clinical trial will have to tell. It may be given in a low dosage which could significantly reduce costs.
MAbCo19 was administered to the first patient in Verona on Monday. In phase 1, 30 healthy volunteers will participate. Subsequent phases will include a few hundred patients with Covid-19.
Even though the first vaccines and treatments have already been approved, these and other new candidates are still needed.
Covid-eVax has several advantages over the currently approved vaccines. DNA can be produced cheaply and on a large scale. It does not require ultra-cold storage which makes it easily applicable also in regions with weak health infrastructure. It does not induce immunity against the viral vectors used by other vaccines. Above all, its flexibility allows the developers to adapt quickly against new variants of the coronavirus. “We are already testing vaccines against the variants from Great Britain, South Africa and Brazil”, Aurisicchio says. “In a timeframe of two weeks we can generate a new vaccine based on potential new variants that may emerge.” Takis has an algorithm that can predict which variant is probable to emerge over time.
Even when we have vaccines, treatments are still needed as well. The importance of the role monoclonal antibodies will play in the collective efforts to fight COVID-19 depends on several factors. First, the efficacy of the vaccines. Second, their availability – just recently, amidst reports of vaccine production delays Germany has ordered 200.000 doses of antibody treatments. Third: There will be people who cannot or do not want to be vaccinated. For those antibody treatments that come with a high safety profile may be a solution.
After all, mAbCo19 could prove to be more than a treatment. The candidate will be now tested for therapeutic use. However, it is also a candidate for prophylactic use. “At the moment this use is not yet being investigated”, Baccheschi says. “But it could be done soon.”
Both candidates might be instrumental for a long-term solution in the battle against the pandemic. Until then there are still some steps to go.
For Covid-eVax, phase 1/2 trial results are expected around summertime. Plans for a Phase 3 study are yet to be made concrete. A challenging task. Not only because it has to be seen how the pandemic evolves. Circumstances for Phase 3 studies have changed. “Now that many vaccines are available it may be unethical to have a placebo group”, Aurisicchio explains. Takis may have to work with a fewer number of patients and ask for a conditional approval by the EMA. Another option would be a “human challenge trial”, a trial involving the intentional exposure of the test subject. In the UK young volunteers are challenged with SARS-CoV-2 in a controlled environment for research purposes. Once the experimental conditions are established, the human challenge trial could also be a method to test the efficacy of COVID-19 vaccines. But Takis and Rottapharm Biotech are still weighing the options. “It’s too early to have a clear picture”, Aurisicchio says.
The mAbCo19 Phase I clinical trial will be paired with a phase II/III trial a month from now. “With this approach we aim to have the product registered by the end of Spring”, Baccheschi says.
March 4, 2021 by kENUP