Vaccine skepticism in Germany multiplies with novel COVID vaccines
photo: stilistica.design based on data provided by Civey GmbH
Today, the results of a first representative empirical study of COVID-19 vaccine skeptics, conducted by kENUP Foundation, the Swiss Academies of Arts and Sciences and the EIB Institute, have been published.
- 13.7 million new vaccine opponents since pandemic onset;
- skepticism mostly exacerbated by fears of long-term damage and fast vaccine approval processes, correlates with corona virus denial;
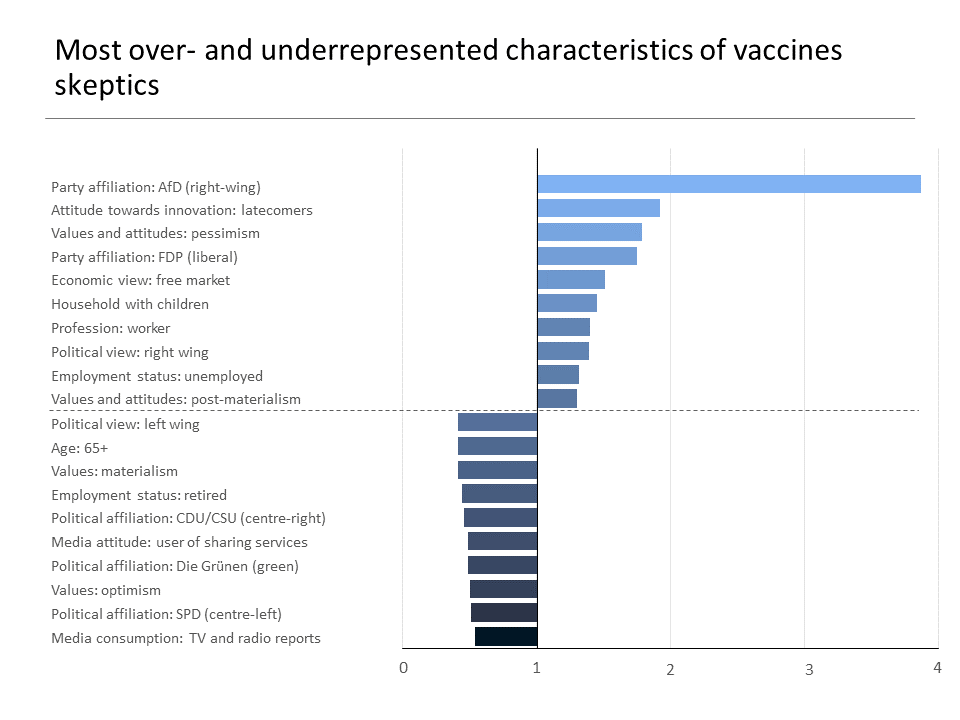
- 90% of skeptics feel they have no influence over politics, are predominantly AfD-leaning and about 45% attained the lowest level or left secondary school without certification;
- while new skeptics could be convinced by facts, long-term anti-vaccinationists appear unyielding.
The first representative study of COVID-19 vaccine skeptics, conducted by kENUP Foundation, the Swiss Academies of Arts and Sciences and the EIB Institute, identified the socioeconomic and consumer behavior profile, as well as the dominant values and positions of COVID-19 vaccines skeptics and examined potential strategies to increase willingness to get a COVID-19 vaccine in that cohort.
Vaccination is one of the public health measures that has had the greatest impact on the reduction of the burden from infectious diseases. According to the World Health Organization, vaccines prevent up to 3 million deaths worldwide each year. Vaccines are the most important tool in exiting the ongoing COVID-19 pandemic. Yet, anti-vaccination sentiments are highest in Europe compared to other regions in the world, potentially undermining vaccination and herd immunity efforts.
Overall, 19.6% of the German population make up the COVID-19 vaccine skeptic group, those unlikely or very unlikely to take the jab once offered to them. Almost 84.2% of that group were not opposed to vaccines before the corona crisis. This means that 13.7 million new skeptics are contributing to the high COVID-19 anti-vaccine sentiment.
COVID-19 vaccine skepticism is mainly driven by the fear of long-term damage (66.0%), the fast approval process (54.7%) and fear of adverse health effects (53.0%). It correlates with corona virus denial: 21.7% of vaccine skeptics and 36.8% of long-term anti-vaccinationists disagree that COVID-19 causes serious health problems. Denial that the virus has infected many people worldwide is 21.8% and 40%, respectively for new skeptics and long-term anti-vaccinationists.
While 69.3% of all vaccine skeptics claim that they could be convinced to take the COVID-19 vaccine through factual assurances, 69.4% of long-term anti-vaccinationists state that nothing would convince them. On the assurances needed for attitudinal change within the overall cohort, exclusion of long-term damage (52.0%), proven effectiveness (40.8%) and the evidence that genes are not changed (32.0%) top the list. Moreover, neither celebrity endorsement nor prospects of long-distance travel are convincing skeptics to be vaccinated. Vaccine skeptics seem to have more trust in vaccines originating from Germany, the United States, and the United Kingdom. Vaccines from Russia, China and especially India would have low chances to convince skeptics.
A prevailing lack of life meaning and political participation defines the cohort of vaccine skeptics: 89.2% feel that they have no influence over politics. Of the long-term anti-vaccinationists, 35.2% express existential fears and financial worries, with life lacking any perspective for 35.6% of them. In terms of political affiliation, the anti-vaccination group is predominantly AfD-leaning (43.0%, far-right), with support of FDP (liberal) and Die Linke (left) also over-represented, while affinity to SPD (centre-left), CDU/CSU (centre-right) and Die Grünen (green) remains significantly underrepresented. Anti-vaccination sentiments are much higher in people of non-Christian religions.
Vaccine skeptics predominantly do not work, or if they do, they are not employed but pursue independent activities. 44.8% of them left secondary school without any certification or attained the lowest certification level. The group predominately lives in households with children and in low density areas with lower purchasing power, with a strong spike in the Eastern German States.
The classical news channels, including television and curated authoritative content on the internet, do not reach the anti-vaccination group, as their media consumption is dominated by YouTube and Facebook. On the other hand, they are predominantly late adaptors to technology. The group is further characterized by a high affinity to brands, having fun with friends, DIY, and a low kinship to eco-friendly products.
To access the full study, please click here.
The partner's press release can be accessed here.
To access kENUP's businesswire release, please click here.
Governments spent at least €93bn on COVID-19 vaccines and therapeutics during the last 11 months
photo: stilistica.design
- New figures from kENUP Foundation show extent of public investments in COVID-19 vaccines and therapeutics
- 95% of funds in support of vaccines, with just 5% allocated to therapeutics
- Most funding was granted through a novel mechanism: 93% of all public funds were committed through advance market commitments (AMC), e.g. by Operation Warp Speed in the United States, or by the European Commission
- Geographically, 32% of public funds came from the United States, 24% from the EU and its Member States, and 13% from Japan and South Korea
- On the vaccine side, 71% of public funding went into products under development by SMEs and MidCaps, with only 18% deployed to large pharmaceutical manufacturers
According to analysis conducted by kENUP Foundation, the public sector has dedicated at least €93bn to COVID-19 vaccines and therapeutics in 2020. More than 95%, about €88.3bn, was spent on vaccine companies. Only 5% of public COVID-19 funds were spent on therapeutics.
Most of the funds, around €86.5bn, were used to conclude advance market commitments (AMCs). In return for the right to buy a specified number of vaccine doses in a given time frame, governments finance part of the upfront costs faced by vaccines producers in the form of AMCs. Just 7% of funds were spent through preferred loans or conventional grants.
The data also shows the origin of the funding: 32% of funds directed towards vaccine producers come from the US, 24% from the EU, and a total of 13% from the governments of Japan and South Korea.
For vaccines, exceptionally, governments invested 71% or at least €63.1bn into Small and Medium Enterprises (SMEs) and MidCaps. Only 18% of funds went to big pharma, underlining the importance of SMEs in driving innovation.
Holm Keller, Chairman of kENUP Foundation said: “Public investments have been instrumental in supporting innovation in the fight against the coronavirus. To bridge the time until broad rollout of vaccines, further investment in therapeutics is especially important. In parallel, a dedicated public pandemic preparedness scheme that would make vaccines and therapeutics readily available at lower development costs for any kind of pandemic pathogen is needed”.
The figures are kENUP’s analysis of publicly available information. They do not include private sector investments. The data was correct on Sunday January 10, 2021.
Please click here for kENUP's press release.