VPM1002
is a novel, recombinant BCG vaccine for the prevention of COVID-19 via the induction of trained immunity.
Under development by Serum Institute of India Pvt. Ltd., Bilthoven Biologicals B.V. and Vakzine Projekt Management GmbH (VPM), in cooperation with the Max Planck Institute for Infection Biology, Berlin.
On August 27, 2020 the European Investment Bank has announced
it is backing VPM1002 with an investment of € 30 mio.
VPM1002
Serum Institute of India Pvt. Ltd. - the world's largest manufacturer of vaccines by numbers of doses - alongside its European partners propose to leverage Bacille Calmette-Guérin (BCG) vaccine’s beneficial heterologous (“off-target”) effects and proven antiviral and immune modulatory properties that protect against infectious diseases other than tuberculosis.
BCG is an attenuated live vaccine introduced into clinical practice in the 1920s. It is the most widely used vaccine worldwide and has a strong safety profile. BCG-induced innate immune training reduces experimental human viremia with RNA viruses, and in animal models reduces virus-associated death. Randomized trials in older adults and observational studies in children show that BCG markedly reduces acute respiratory infection. BCG is well known as a profound stimulator of innate immune responses. It induces genome-wide epigenetic reprogramming of innate immune cells leading to long-term functional changes and modulation of immunity. The World Health Organisation (WHO) has called for randomized trials to evaluate BCG’s off-target effects. VPM1002 is an improved recombinant BCG with the M. bovisurease C gene replaced by the listeriolysin O-encoding gene hly. This further enhances innate immunity and induces strong T cell responses. Compared with other types of BCG, VPM1002 has more potent heterologous effects which could be demonstrated in clinical trials in the context of bladder cancer immunotherapy. Extensive safety data in adults and children have been published. Most importantly and unlike other BCGs, VPM1002 is produced with more uniform inter-batch reliability using methods that allow rapid scalability to billions of doses required to meet immediate global need. Taken together, characteristics of VPM1002 that include 1) safety, 2) more potent protective heterologous effects and 3) scalability, poses VPM1002 to be a promising intervention against SARS-CoV-2.
The COVID-19 pandemic is rapidly worsening in all parts of the world, overwhelming health systems with demand for care that exceeds deliverable capacity and is compounded by high disease rates among healthcare staff. VPM1002 may act to ameliorate disease severity and mitigate transmission. Even moderate individual efficacy can have dramatic impact at population level through direct (reducing severe disease burden on health systems) and likely through indirect (transmission reduction) influence on pandemic progression. Hence, it can contribute to sustained health systems during this rapidly increasing crisis by offering a safe, affordable and available vaccine. Investment in large scale manufacturing will depend on strong evidence of efficacy from randomized evaluation and would be a worthwhile global effort even if superseded by a specific SARS-CoV-2 vaccine. The scale up of delivery programs for an interim vaccine such as BCG would still be a very useful infrastructural investment for more rapid roll-out of a subsequent definitive vaccine. There seems little to lose in terms of safety or opportunity cost, and potentially much to gain.
Based on previous experience and randomized controlled trials in adult and elderly individuals, the risks of VPM1002 vaccination are considered low. The objective of the ongoing clinical trials is to evaluate the beneficial effects of VPM1002 vaccination through a lower work absenteeism rate of HCP and/or a mitigated clinical course of SARS-CoV-2 disease and other infectious diseases in HCP and the elderly.
Vaccine recruitment is getting back on track as the number of Covid infections increases again
our insight of the week - October 3, 2020
Town Hall, City of Hannover, Germany, hometown of VPM, photo adobe stock
The ups and downs of the past months of the pandemic were also reflected in research projects focused on a vaccine against Covid-19. When Dr. Leander Grode started the main recruitment of volunteer participants for a study in June and July, those happened to be the two months with the lowest number of new Covid cases in Germany.
“A lot of people thought the pandemic might be over”, Grode explains on video call from his office in Hannover. “People were discussing openly if scientists had overreacted and people had just started to panic.” That made it a lot more difficult to find volunteer participants for the trial.
The trial is testing VPM1002 as a possible vaccine against Covid-19. It is not meant primarily to prevent infection, but stimulate the immune system and minimize the impact of the disease.
Now, the numbers of infections are back up, and in some German states and cities it is just as high as in March and April, the previous peak of the pandemic. “Sadly, due to those rising numbers, people in Germany are again more aware of Covid”, Grode says. He hopes to turn this negative development into something positive by advancing the recruitment of volunteers to help fight the virus with a possible treatment.
Swaminarayan Temple, Pune, India, hometown of Serum Institute of India, photo adobe stock
VPM1002 could also have a positive effect on the severity of an influenza infection, which otherwise can become dangerous for the elderly. For the BCG vaccine, the predecessor of VPM1002, these positive effects have already been proven. Many of the elderly that have volunteered for the trial still know BCG from their childhood when it was first introduced as a vaccine against tuberculosis, Grode explains. They know that this type of vaccine has been successfully used in the field for many decades.
Grode expects they will finalize their recruitment of 2100 elderly participants in November and get the final results around six months later, in May or June 2021. Until then, all the participants will be monitored using a remote system, a web portal where they can give daily and weekly updates of their status.
This remote system is also a way to prevent scientists to learn too much about the results of the study before it ends. This is an ethical requirement for clinical trials to avoid bias. The only information that gets looked at straight away is when there would be severe side effects like a fever. “Luckily, so far we have very few reports of any side effects”, Grode says. “I’m happy everything is going so smoothly.”
While Grode and his team are building their volunteer base, the Serum Institute of India, which they collaborate with, has already completed their recruitment of 6000 people. This is linked to how severely the country was hit by the pandemic, Grode explains. So far, there have been over six million cases. “We hope we get results from the colleagues in India as soon as March. So far, they are really positive”, says Grode. “But we will have to wait for the data.”
October 3, 2020 by kENUP Foundation
Prof. Stefan Kaufmann : an immune booster vaccine for Covid19, and the next pandemic
our insight of the week - June 19, 2020
Prof. Stefan Kaufmann. photo courtesy Max Planck Institute for Infection Biology
Before the first volunteer participant was immunized with VPM1002 as a potential vaccine against Covid-19 three weeks ago, the research into the candidate took a lot of unexpected turning points. There is one person who knows exactly how many: Prof. Dr. Stefan Kaufmann, emeritus director and founding director of the Max-Planck-Institute for Infection Biology and professor at Charité in Berlin, who dedicated a large part of his renowned career to VPM1002.
Over the course of his career, a lot of pieces of the puzzle that is our immune system have been moved around, with researchers all over the world trying to understand its mechanics better. A few breakthroughs have paved the way for how VPM1002 is expected to be effective against Covid-19.
Generally speaking, there are two important pillars of the human immune response, Kaufmann explains on video call from his office in Berlin. The innate or non-specific immune response and the acquired or specific immune response. The innate immune response operates through cells of our immune system which have no specific receptors for antigens. “Once they are activated, they will attack any pathogen that is around them”, says Kaufmann.
Then there is the specific immune response which works through cells with receptors that can very specifically recognize bacterial or viral antigens. They only get activated when they recognize their specific antigens.
For a long time, most scientists, Kaufmann included, assumed that the specific immune response is the most important pillar of our immune system, in a way steering all the other parts.
illustration courtesy Max Planck Institute for Infection Biology
Ten years ago, there were breakthrough findings suggesting that the non-specific immunity actually is more important and, in many ways, giving instructions to the specific immunity. Today, Kaufmann says, it now seems to be quite clear that they are both equally important and inter-related.
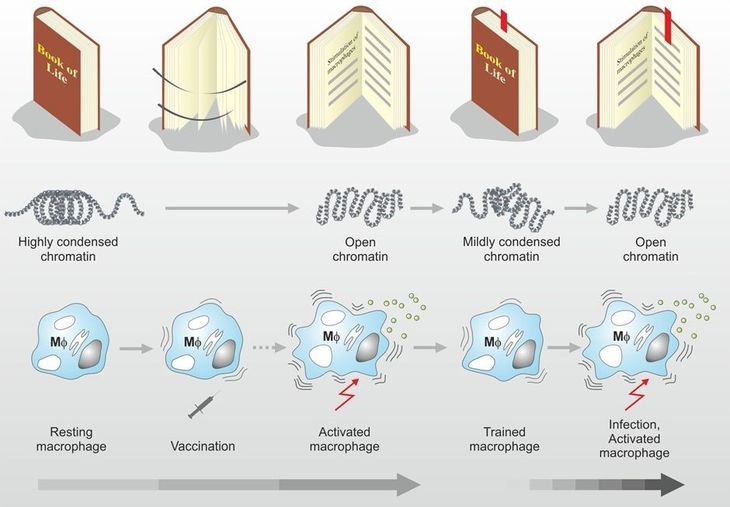
Another critical finding that paved the way for VPM1002 was that once the innate immunity is activated, even though at some point it goes back to being inactive, it always remembers stimuli that activated it in the first place. When the same or another pathogen enters the body, it can respond more rapidly. That is what scientists call trained immunity. “Because it can be compared to training a muscle”, says Kaufmann. This training also takes place when someone receives a live vaccine, like VPM1002.
On top of those basic research findings it was also observed that in low income countries, where a lot of babies were immunized with BCG, the overall mortality was lower beyond death due to tuberculosis. This could also be explained by a trained immune system. So, it was this combination of research findings and observations in the field that now creates enormous hopes for VPM1002 against Covid-19.
“We think this could be an ideal candidate as an intermediate vaccine for people at risk”, says Kaufmann.
VPM1002 is now being tested carefully. Previous research with bladder cancer patients showed that VPM is much safer than its predecessor BCG: almost 50 percent of the patients who could not be treated with BCG showed a better reaction to VPM1002. That is why Kaufmann is optimistic towards the outcome of the Covid-19 trials. He hopes that VPM1002 might be available in time for a second wave of infection to protect the people at the forefront of this pandemic.
And, Kaufmann adds, the non-specific immune reaction induced by VPM1002 could also be helpful in fighting the next unknown virus. If it is active against Covid-19, it is also likely to work against others, says Kaufmann. “That’s not today’s or tomorrow’s issue, but we have to plan ahead this time.”
June 19, 2020 by kENUP Foundation
Prof. Stefan Kaufmann. photo courtesy Max Planck Institute for Infection Biology
New TB vaccine boosts immune system to fight off COVID-19
our insight of the week - May 29, 2020
Team of Vakzine Projekt Management GmbH (VPM) - Photo Courtesy VPM
Finding a vaccine or a treatment against the new coronavirus is a race against time, but thankfully a lot of promising projects are already very advanced. This week, the first volunteer participated in a clinical trial of the vaccine candidate VPM1002 in Hanover, Germany.
In the first trial, 1200 hospital professionals will be participating, followed by a second trial two weeks later, targeting approximately 2100 elderly people.
“The idea that this candidate could be used as a vaccine against respiratory diseases is quite old”, says Dr. Leander Grode from Vakzine Projekt Management GmbH on a video call from his office in Hanover. “But sometimes you need an extreme situation like this to get fast approval.” Grode is coordinating the clinical trials and has been working on VPM1002 since 2000; at the beginning as a scientist at the Max-Planck-Institute for Infection Biology in Berlin and later since 2004 in cooperation with the group of Prof. Dr. Stefan H.E. Kaufmann from the Max Planck Institute for Infection Biology in Berlin"
Originally, VPM1002 was only developed as a vaccine against tuberculosis (TB) and is being developed in a setting with a high burden of TB (Africa). In 2012 the German team started their collaboration with Serum Institute of India Pvt. Ltd. (SIIPL), one of the biggest vaccine manufacturers in the world, with plans to make the promising TB vaccine available to all newborn infants who are at risk of infection, mostly in poorer countries in South East Asia and Africa.
Getting funding for research concerning illnesses that barely affect Western countries, like TB, is often a long and complex process. But finally, Grode was getting closer to setting the Phase III trial for VPM1002 in motion. But at this fateful moment, the coronavirus pandemic hit, and all eyes were now on a new vaccine.
From the start there were a few indicators that suggested that VPM1002 could be a good candidate in the fight against COVID-19. BCG, the original immunization against TB, is the most used vaccine in the world and also the predecessor of the VPM1002 candidate. This old TB vaccine has not changed much in the past 100 years, but is known to cause several side effects. VPM1002 was developed as a safer alternative to BCG with significantly less adverse reactions.
BCG has been shown to help against viral respiratory infections in general, like influenza. When Grode learned that BCG was being tested for use against COVID-19 in the Netherlands, he jumped on the opportunity to initiate clinical development for VPM1002 in this context as well.
The goal was to conduct two Phase III clinical trials, one with healthcare professionals and one with elderly people. If these studies obtain positive results, VPM1002 would be allowed to proceed into market authorization right away. By the beginning of May, the German authorities gave their approval after an extremely fast but thorough review process.
There is an important distinction between BCG or VPM1002 and other potential vaccines that are currently under development: VPM1002 and BCG are immune stimuli, which means that they do not prevent the infection as such, but minimize the impact of respiratory disease like COVID-19. “We hope it strengthens the immune system, so the severity and the duration of the disease are reduced”, Grode explains. If proven successful, VPM1002 will be a significant weapon in the fight against COVID-19, not only because it boost the immune system, but because it could be used much sooner and more broadly than a COVID-19 specific vaccine, bridging the gap for the people who desperately need it until an effective vaccine is found and manufactured.
One of the main concerns towards the use of BCG against the new coronavirus is that it will take away critical supplies from newborn infants in developing countries, leaving them vulnerable to TB. BCG with its traditional manufacturing methods cannot be produced on a large scale that fast. However, the manufacturing process for VPM1002 is significantly more efficient and scalable. The SIIPL, is able to get five million doses of VPM out of a 30-liter vessel, which also can be scaled up to vessels of 500 or even 1000 liters. “You will have more doses than you can imagine. If it’s successful, they will really bring the vaccine to everyone”, Grode says. “Not just the rich countries.”
A week prior to the trial start in Germany, SIIPL started a similar trial in India. “We will share and compare our data as soon as we have it”, Grode says. A realistic expectation is March 2021, that’s when the first – and final – results will be in, he says. “Being patient will make the results a lot more valid”, says Grode. “Quality needs time.”
May 29, 2020 by kENUP Foundation
Dr. Leander Grode (left) - Photo Courtesy VPM