PLX-PAD
is a cell therapy candidate for the treatment of severe pneumonia due to COVID-19.
PLX-PAD
Pluristem Therapeutics Inc.
(NASDAQ, TASE: PSTI) is a leading regenerative medicine company developing novel placenta-based cell therapy products (PLX). PLX cell products are off-the-shelf, requiring no tissue matching prior to administration. The cells release a range of therapeutic proteins in response to inflammation, ischemia, muscle trauma, haematological disorders, and radiation damage. Pluristem has a strong intellectual property position, Company-owned and operated, GMP-certified manufacturing and research facilities, and strategic relationships with leading international collaborations.
PLX cells are allogeneic mesenchymal-like cells that have immunomodulatory properties that induce the immune system’s natural regulatory T cells and M2 macrophages, and thus may prevent or reverse the dangerous overactivation of the immune system. Accordingly, PLX cells may potentially reduce the fatal symptoms of COVID-19 induced pneumonia and pneumonitis. Previous pre-clinical findings of PLX cells revealed significant therapeutic effects in animal studies of pulmonary hypertension, lung fibrosis, acute kidney injury and gastrointestinal injury which are potential complications of the severe COVID-19 infection. Clinical data using PLX cells demonstrated the strong immunomodulatory potency of PLX cells in patients post major surgery. Taken together, PLX cells’ potential capabilities with the safety profile observed from clinical trials involving hundreds of patients worldwide potentially position them as a therapy for mitigating the tissue-damaging effects of COVID-19.
Pluristem announced in April 2020 preliminary data from its COVID-19 Compassionate Use Program in Israel, treating seven patients with Acute Respiratory Failure. The results demonstrated 100% survival rate for all seven patients. 66% of patients that completed one week follow up demonstrated improvement in respiratory parameters and 50% of the patients that completed 1 week follow up demonstrated advanced stages of weaning from ventilators. In parallel, a compassionate use program has started in the United States next to clinical trials in the US and Europe
“The virus is telling us loud and clear: start to think globally”
our insight of the week - October12, 2020
Louvre Abu Dhabi, Abu Dhabi, hometown of ADSCC, photo adobe stock
The global pandemic has shown, maybe like nothing before, how connected the world is. First, this was demonstrated by a virus traveling across the globe in a matter of weeks, even days. But now, for Yaky Yanay, CEO of the Israeli regenerative medicine company Pluristem, it is time to put this connection to good use.
“This virus is telling us loud and clear: start to think globally”, says Yanay. Therefore, his company Pluristem based in Haifa, Israel, signed a MOU with the Abu Dhabi Stem Cells Center (ADSCC) to collaborate in the development of cell therapies for the treatment of severe diseases including Covid-19.
The MOU follows the Israel-United Arab Emirates peace agreement that was established on August 13th, 2020. The UAE is only the third Arab country to formally normalize its relationship with Israel. With their MOU, Pluristem and ADSCC are on the forefront of this moment in history. “I believe that we as business leaders should build the bridges into peace. Science can bring people together, there is no border and no limitation to health”, says Yanay.
But Yanay points out that he hopes this will not only benefit the two countries directly involved. “I want our work to benefit everyone: the US, Europe, the Middle East, Israel. We want to make a global impact”, he says.
And it is already a global project. In Phase II studies currently conducted in Germany, Israel and the U.S. comprising 180 patients in total, Pluristem uses its PLX-PAD cells to treat Covid-19 patients with ARDS (Acute Respiratory Distress Syndrome) and on mechanical ventilation. Those PLX-PAD cells are derived from a placenta after a full-term delivery.
They induce the immune system’s natural regulatory T cells, therefore may help the patient’s body to regain balance of the immune system. So far, Pluristem has observed very positive responses to the treatment from compassionate use programs, says Yanay.
Yanay also sees a logistic advantage of his product when it comes to battling Covid-19: The PLX-PAD can be manufactured on a large scale. With cells derived from a single placenta, they can treat more than 20.000 patients. Pluristem invested a lot to build the technology and the means to manufacture in such a large scale, Yanay explains.
Haifa, hometown of Pluristem Inc., photo adobe stock
Pluristem wants to combine this orientation towards industrial technology with ADSCC’s scientific and clinical expertise. The ADSCC was founded in March 2019 as the first of its kind in the United Arab Emirates. “It is a big recognition for us that we are the first center in Abu Dhabi to receive the license to work with stem cells”, Dr. Yandy Castillo, immunologist and quality manager at the center, explains.
Only one year after its opening, the ADSCC was able to swiftly react to the Covid-19 crisis. The team designed a clinical trial for treating the new coronavirus in April and was able to start treatment in May. The patient’s own stem cells are first extracted and then reinserted directly into the lungs through inhalation with a nebulizer.
Until today, they have treated 146 patients under the initial clinical trial in Abu Dhabi, with the approval to extend it within the rest of the Emirates, resulting in the treatment of more than 3300 patients up-to-date, as Castillo explains.
When bringing those approaches together, Yanay hopes they will be able to learn more about how the cells respond best during the different stages of a Covid-19 infection. The collaboration will also lead to multiple joint projects, benefitting of each company’s expertise.
The first project will combine PLX placenta cells from Pluristem with the nebulizing technology used by ADSCC to treat Covid-19 patients. Instead of an injection, they will get the PLX cells through inhalation. “We know that our cells have the capacity to expedite the curing process”, says Yanay, “we are excited to see how the nebulizing technology will enhance this.”
Castillo from the ADSCC could also imagine an exchange that goes beyond joint projects. Both him and Yanay are very open to the idea of personnel exchange between the company in Haifa and the research center in Abu Dhabi. “The center is open for everyone from Pluristem”, says Castillo.
Their collaboration was called forth by battling Covid-19, but Yanay hopes that this is just the beginning, paving the way for future collaborations. But not just for Pluristem and ADSCC. “I am very confident that other research projects all over the world will follow with more and more collaborations”, says Yanay.
October 12, 2020 by ACUMEN
Rapidly lowering COVID-19 mortality and cost to society by means of a powerful therapeutic?
our insight of the week - July 31, 2020, update August 6, 2020
Pluristem plant in Haifa, Israel, photo courtesy company
Time to catch up again with Yaky Yanay, CEO of regenerative medicine company Pluristem, and take their pulse after it secured € 50m financing from the European Investment Bank back in April.
The world today is looking for a breakthrough to cope with the Covid-19 and the Pluristem’s PLX-PAD cells that are derived from placenta are currently being examined through clinical trials as part of the global effort to potentially improve the lives and health of people around the world. Pluristem has initiated a Phase II clinical trial for Covid-19 complicated by Acute Respiratory Distress Syndrome (ARDS), enrolling 140 patients in the U.S., with results expected to be published during the fourth quarter of 2020. In addition, the company also applied for a clinical trial authorization in ARDS associated with COVID-19 in Germany.
Parallel to conducting these clinical trials, the company also treats Covid-19 patients under compassionate use programs in the U.S. as well as in Israel. One of the treated patients is Edward Pierce, the Broadway designer (Angels in America/Wicked) who recovered from the virus after becoming the first American to be treated with Pluristem’s cells. His case and recovery have now entered history: Pierce has called this treatment a true miracle — he went from near death to leaving the hospital in short order.
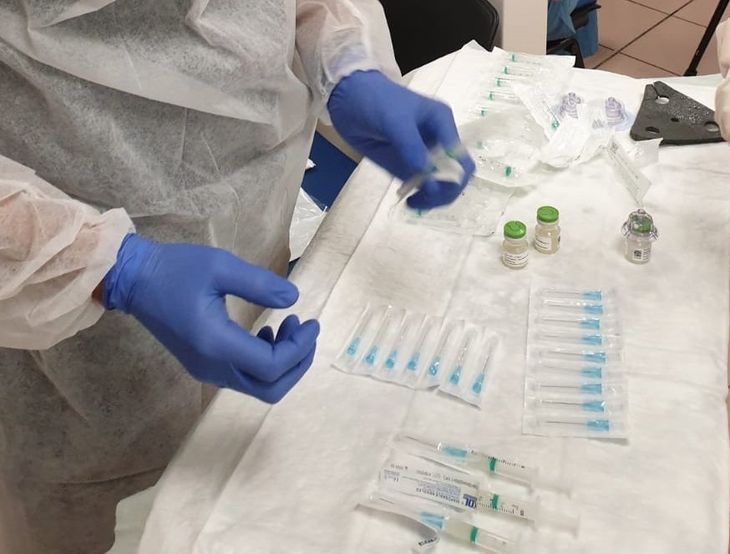
Yaky Yanay (right) with Pluristem staff, during preparation for compassionate use in Israel, photo courtesy company
“We are looking to enroll those with the most severe conditions because we want to save their lives,” Yaky tells us. “That’s why we are heading to the COVID-19 hotspots of the U.S. Thanks to our unique cold chain capacity, the PLX-PAD product is ready to use on patients after just a few hours notice.” The products are easy to transport and administer in large supplies, giving them a particular advantage for marketing and distribution and the potential to save the lives of thousands of patients undergoing mechanical ventilation like Mr Pierce.
In Europe, Pluristem has established a new wholly owned subsidiary, based in Berlin, built on the long-standing relationship with its university hospital Charité. In Germany, the enrollment of patients into the EU-arm of Pluristem’s Phase II is envisioned to start immediately once the necessary clearances have been received.
Over and above its work on COVID-19, Pluristem runs a Phase III Critical Limb Ischemia (CLI) Study with top line interim data analysis results available in fourth quarter calendar year 2020, a Phase III Muscle Regeneration following Hip Fracture Study with results published in third quarter of 2021 as well as a Phase I Hematopoietic Cell Transplantation (HCT) Study to be published in the first quarter of 2021.
Pluristem staff preparing for an injection during compassionate use in Israel, photo courtesy company
Pluristem’s CEO believes that what governments can do is to make more therapies available to save lives and counter-balance their perceived focus on vaccination as the primary solution – especially since it may take time until a safe and effective vaccine may become available in the necessary quantities, while a powerful therapeutic may be around the corner. The key aspect of regenerative therapies is that they mirror the body‘s own healing abilities. ”The PLX-PAD cell therapy will not aim to kill a virus but boost the immune system's ability to fight it on its own,” says Yaky.
With $59m in cash and cash equivalents and its EIB financial backing, assuming all milestones achieved, Pluristem believes it can keep up its operations for at least three years. So, finishing the conversation with a warm flourish, Mr Yanay extends an open-ended invitation to visit the laboratories in Haifa: “We hope you will be able to visit us soon!”
July 31, 2020 by ACUMEN
Treating COVID-19 with placental expanded cells
PLX-PAD
- our insight of the week - May 22, 2020
Courtesy Jeff Rhodes, Holy Name Medical Center
“I honestly feel like it was the Pluristem treatment that gave him the edge on everybody else, and what did it for him. Really, honestly, it’s nothing short of a miracle.”
Patient’s wife in The Daily Beast on May 21 2020
Recently, Broadway star-designer Edward Pierce recovered from COVID-19 after being treated with Pluristem’s experimental PLX-PAD drug through the FDA’s compassionate program designed for patients with no real hope for survival. His amazing recovery attracted wide media coverage, shedding light on the fact that effective therapeutics may be much closer at hand than a vaccine against COVID-19.
This story, though, is currently only one story, with the underlying data being indicative but not yet statistically relevant. Moreover, what may look like a miracle to patients and their relatives in such distressful, presumably terminal situations is not divine intervention but a mid-point in a 20-year long journey of scientific rigor. This expedition begun at two leading global research institutions - Technion Israel Institute of Technology, and Weizmann Institute of Science– with the aim to treat and heal diseases that are assumed to be chronic.
Courtesy Pluristem Therapeutics Ltd.
“We need smarter medicine, otherwise humanity will end up bankrupt” says Yaky Yanay, CEO and President of Pluristem. “We cannot continue treating patients in the chronic way and keep them in hospitals, we need a shift of paradigm”. Yaky Yanay and his team are strong believers in the regenerative power of placental cells. The company directs much of its energy at supporting aging populations and giving them an opportunity to enjoy a high quality of life – likely to be a key major post-Covid-19 societal objective.
Pluristem has been improving the medical offer for more than a decade under highly controlled conditions. The Company is in several clinical studies for treating diabetes comorbidities, accelerating muscle recovery in hip replacement and even dipping into recovery from nuclear radiation. Yaky Yanay calls the product “a native speaker of the body”. When the Placental eXpanded (PLX) cell is injected in the body it responds to the signals produced by the inflamed area and further stimulates a regenerative process. With a single cell potentially treating around 20000 patients, this has significant potential for current healthcare therapies and treatments. The PLX cells derived from human placenta make them literally a therapy that was born to protect.
As the COVID-19 outbreak started to spread in China, the Pluristem team began analyzing the effects of the virus on patients. They noted that many were dying from pneumonia, high levels of pulmonary inflammation and a complete and severe auto-immune reaction. The company had already developed a model to support the treatment of pneumonia with PLX cells so Pluristem knew exactly what was required. As soon as it got approval from Israel's Ministry of Health it set up a compassionate-use program in its home country and under the FDA Single Patient Expanded Access Program it started to treat patients also in the U.S.
Courtesy Pluristem Therapeutics Ltd.
In their latest update
Pluristem highlighted outstanding results from the 28-day follow up on patients that were on invasive mechanical ventilation and treated with PLX cells. The survival rate of 8 patients in critical condition was 87.5% while 75% were taken off the mechanical ventilation and 62.5% could leave the hospital. More results are expected however this first set of data marks a potential way of improving the survival rate of COVID-19 patients.
The EIB recently signed a €50M financing agreement
with Pluristem that will support the research and development activities, expansion and commercialisation of its innovative regenerative cells system also in Europe. PLX cells for COVID-19 has been cleared for Phase II clinical trials in the U.S
ready for the first patient to be treated and is now awaiting the green light from the Paul Ehrlich Institute for initiation of the trial in Germany and Italy. CEO Yaky Yanay points to his new partners: “I am grateful to have the support of the European Investment Bank (EIB) and kENUP Foundation. They believe in our vision and understood that a quick reaction was needed.”
May 22, 2020 by Acumen, with additional text provided by kENUP
PLX Cells for COVID-19
PLX cells
are available off-the-shelf and once commercialized can be manufactured in large scale quantities. Pluristem believes its PLX cells will offer a key advantage in addressing the COVID-19 global pandemic. PLX cells are allogeneic mesenchymal-like cells that have immunomodulatory properties that induce the immune system’s natural regulatory T cells and M2 macrophages, and thus may prevent or reverse the dangerous overactivation of the immune system. Accordingly, PLX cells may potentially reduce the incidence and/or severity of COVID-19 pneumonia and pneumonitis leading hopefully to a better prognosis for the patients. Previous pre-clinical findings of PLX cells revealed therapeutic benefit in animal studies of pulmonary hypertension, lung fibrosis, acute kidney injury and gastrointestinal injury, which are potential complications of the severe COVID-19 infection. Clinical data using PLX cells demonstrated the strong immunomodulatory potency of PLX cells in patients post major surgery. Taken together, PLX cells’ potential capabilities with the safety profile observed from clinical trials involving hundreds of patients worldwide potentially position them as a therapy for mitigating the tissue-damaging effects of COVID-19.
Mr. Yaky Yanay
was appointed as President from February 2014 and as CEO from June 2019, after a period of being Co-CEO since March 2017. Mr. Yanay served in variety of executive positions in Pluristem since 2006 including Chief Financial Officer, Chief Operating Officer and Executive Vice President. Mr. Yanay is the former Co-Chairman and current board member of Israel Advanced Technology Industries (IATI), the largest umbrella organization representing Israel’s life science and high-tech industries. Mr. Yanay founded over the years several activities and organizations to promote and support the Israeli life science industry. Before joining Pluristem, Mr. Yanay was the Chief Financial Officer of Elbit Vision Systems Ltd. a public, machine vision, high-tech company. Prior to that Mr. Yanay served as manager at Ernst & Young Israel. Mr. Yanay holds a bachelor’s degree with honors in business administration and accounting and is a Certified Public Accountant in Israel.